The statistics behind the Infected Blood Inquiry: an explainer
The Infected Blood Inquiry was established to examine the circumstances under which patients treated by the NHS between 1970 and the early 1990s received infected blood and blood products. Statistics was a key aspect of the inquiry, and it was supported by a team of statistical experts (the Statistics Expert Group), including RSS fellows. Having since published its final report, here the group provides an explainer about the estimates of the number of people infected and subsequently dying from their infection.
The use of contaminated blood products between 1970 and 1991 has been referred to as
“the worst treatment disaster in the history of our NHS”. The timeline is important, covering a period in which a series of viruses were discovered and screening programmes were subsequently established for the UK’s blood supply. Specifically, hepatitis B virus (HBV) was discovered in 1968 and screening set up from 1972; human immunodeficiency virus (HIV) was discovered in 1983 and screening set up from October 1985; and hepatitis C virus was discovered in 1988 and screening set up from September 1991. Before these dates, blood used in a transfusion, or blood products used for patients with bleeding disorders, could not be screened for the respective bloodborne infections.
Moreover, the UK was not self-sufficient in the novel blood products used to treat patients with bleeding disorders. Consequently, blood products made from pooled plasma donations were imported especially from the USA where, unlike in the UK, donors were paid for their donations. As a result, blood products used to treat patients with bleeding disorders were a major route of transmission for HIV and hepatitis C. Blood transfusions in the UK prior to September 1991 were a route mainly for hepatitis C infections. Consequently, a number of people became infected with and subsequently died from these viruses, leading to many tragic stories of families torn apart by illness, stigma and death.
The Infected Blood Inquiry was commissioned by the UK government in 2017
to examine the circumstances surrounding infection from blood and blood products in the UK. While fully acknowledging the importance of individual experiences, it is also important to establish the magnitude of the harm done, and ask:
how many people were infected and died as a result of treatment with contaminated blood, either via blood products or blood transfusions? A
Statistics Expert Group (SEG) was convened to help the inquiry shed light on these vital questions.
The task was broken into sub-questions by the Inquiry, each considering the number of infections and deaths by infection, specifically from HIV, hepatitis C, variant Creutzfeldt Jakob disease (vCJD), which was discovered in 1996 but for which there is no screening test in blood (so that other precautions had to be taken to protect the UK blood supply), and hepatitis B, each from blood products or transfusions.
Quantifying the extent of these infections from blood products or blood transfusions was critical, both to recognise and document formally these events and to estimate how many may have been infected but remain undiagnosed.
After close examination of the available evidence (see below), the group produced estimates of the number of people infected via blood products or blood transfusions for each virus in the UK across 1970–91 (see table). They estimated that 2,900 persons (uncertainty: between 1,700 and 4,700) died up to the end of 2019 as a direct result of their infection. Estimates are provided with an assessment of uncertainty, and a judgement made of the confidence of the statistical experts in their ability to answer the questions posed using the available evidence. Note that the SEG had such low confidence in being able to quantify hepatitis B infections that no numerical assessment was provided.
These
results were presented to the inquiry in September 2022, with senior members of the SEG answering questions on these results from Inquiry lawyers in November 2022. A subsequent report estimated the number of attributable deaths, using a weighted average of a range of plausible models.
Table: main estimates from the reports of the Statistics Expert Group
| Group |
Number of infections
(from main SEG report) |
Number of attributable deaths
(from supplementary SEG report) |
| Estimate |
95% uncertainty interval |
| HIV infections in people with bleeding disorders |
Around 1,250
(moderate/high confidence) |
820 |
730 to 910
|
| Hepatitis C infections in people with bleeding disorders (excluding those with HIV) |
2,400 to 5,000
(low/moderate confidence)
|
350 |
255 to 590 |
| HIV infections in transfusion recipients |
79 to 100
(moderate confidence) |
35 |
32 to 40
|
| Hepatitis C infections in transfusion recipients |
~ 26,800
(95% interval
21,300 to 38,800)
(moderate confidence)
|
1,640 |
550 to 3,440
|
| 10-year survivors after chronic hepatitis C infection as transfusion recipients |
~ 8,120
(95% interval
6,330 to 11,900)
(moderate confidence)
|
| Variant Creutzfeldt Jakob Disease (vCJD) infections from blood and blood products |
5
(high confidence) |
5
|
3 to 8 |
| |
|
|
|
| Total |
|
2,900* |
1,750 to 4,650
|
* The methodology entails that the total is not precisely the sum of the individual estimates
Since recipients of blood transfusion typically experience relative high mortality in the first decade after blood transfusion (for reasons related to the illness which occasioned their need for blood transfusion), the group estimated the number of chronically hepatitis C-infected transfusion recipients who survived at least 10 years after their blood transfusion – see table. Another reason is that chronic hepatitis C infection has a long incubation period until long-term effects become clinically apparent, and so most deaths occur more than 10 years after chronic infection.
Statistical approaches: Most of the questions posed to the expert group could be answered by analysing databases containing information about recipients of blood products or persons who were HIV-infected during transfusion, due to the relatively short period between HIV infection and symptoms for this virus. Multiple sources of information were available providing some opportunity for triangulation, although precise estimates were still not possible due to limitations in the available data sources.
However, there is no established data source for the number of hepatitis C infections and related deaths from blood transfusions, and so a complex multi-stage statistical model was required to bring together the available evidence and estimate the quantities of interest. The SEG’s model was structured as six ordered subtasks representing the whole pathway from infection to the current status of individuals, with the outputs of each task becoming the inputs into the next task (see figure below). They took advantage of the fragmentary available data, taken from a wide variety of sources, combined with evidence-based assumptions about the process of infection and the consequences of acquired disease.
For example, for one subtask, the experts analysed the trends in the number of (all) blood transfusions for the years where this count was available (from 1975) and used these trends to estimate what the number of transfusions would have been in 1970–74 for which there were no data available. For another subtask, they conducted extensive analysis of the Office for National Statistics life tables to estimate the probability of an average individual in the population dying in each year of their study period according to age and sex, before then using estimates from a case-control study of the impact of chronic hepatitis C infection on transfusion recipients to adjust the probability of death for individuals infected.
Models are always imperfect representations of reality and, as such, there is uncertainty surrounding estimates. In this model the experts were careful to propagate the uncertainty derived in each subtask through to the final estimates using Monte Carlo simulation. Uncertainties in estimation are primarily due to a lack of available data, hence the need for sub-modelling to fill the gaps as detailed above. In addition, extensive sensitivity analysis was conducted to assumptions, some of which were necessarily based on subjective expert opinion.
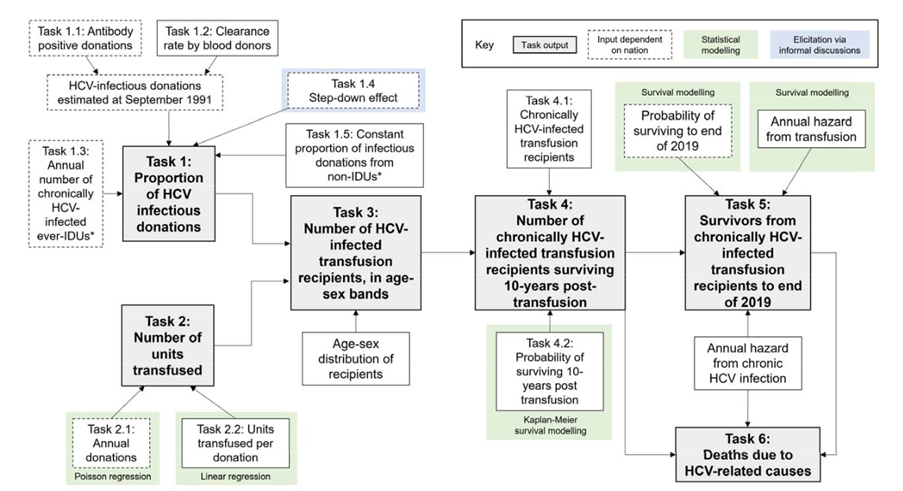 Figure: A schematic version of the model used to estimate the number and outcomes of hepatitis C (HCV) infections in transfusion recipients.
The RSS is grateful to Dr Ruth McCabe, Dr Sarah Hayes, Prof Sheila Bird, Prof Sir David Spiegelhalter, and Prof Christl Donnelly for drafting this explainer.
Figure: A schematic version of the model used to estimate the number and outcomes of hepatitis C (HCV) infections in transfusion recipients.
The RSS is grateful to Dr Ruth McCabe, Dr Sarah Hayes, Prof Sheila Bird, Prof Sir David Spiegelhalter, and Prof Christl Donnelly for drafting this explainer.